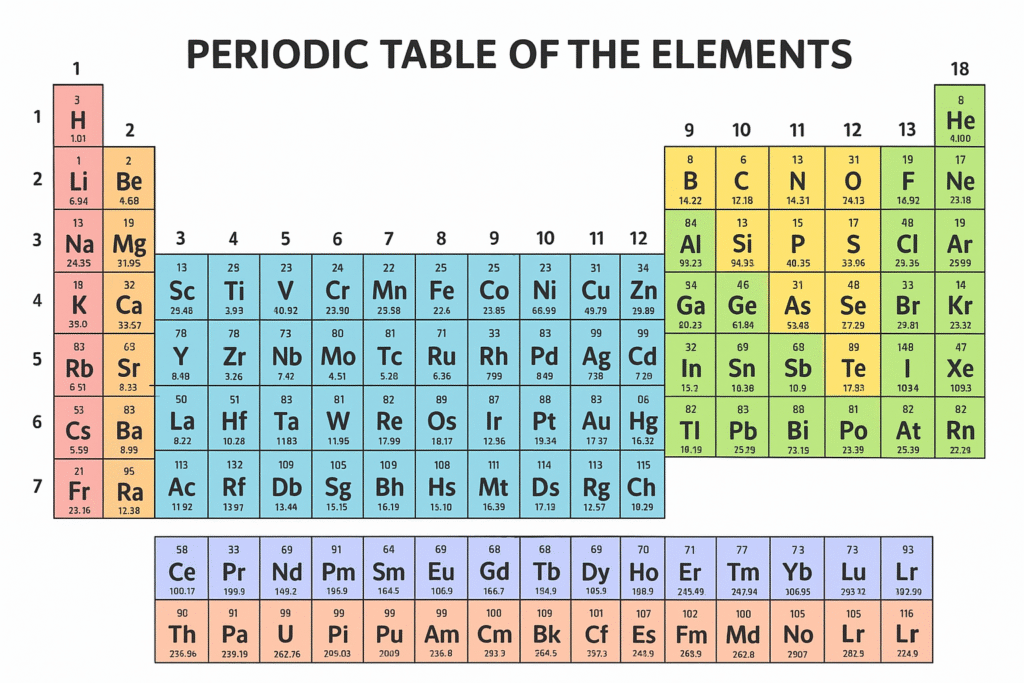
The classification of elements in the periodic table is based on their electronic configuration, properties, and position in the table. Here’s a structured explanation:
1. By Nature of Elements
- Metals → Shiny, conduct heat and electricity, malleable, ductile.
🔹 Explanation: Metals usually look bright (shiny), can carry heat and electricity, can be hammered into sheets (malleable), and can be stretched into wires (ductile). Example: Iron, copper. - Non-metals → Dull, poor conductors, brittle, often gases.
🔹 Explanation: Non-metals don’t shine, don’t carry heat/electricity well, break easily if hammered, and many exist as gases. Example: Oxygen, sulfur. - Metalloids → Show properties of both metals and non-metals.
🔹 Explanation: Some elements behave partly like metals and partly like non-metals. Example: Silicon conducts electricity (like a metal) but is brittle (like a non-metal).
2. By Groups (Vertical Columns in the Table)
- Alkali metals (Group 1) → Very reactive, soft, react with water.
🔹 Explanation: These metals (like sodium, potassium) are so soft you can cut them with a knife, and they react quickly with water to form strong bases. - Alkaline earth metals (Group 2) → Reactive, form bases with water.
🔹 Explanation: These metals (like calcium, magnesium) are also reactive but not as much as Group 1. When they react with water, they form alkaline (basic) solutions. - Transition metals (Groups 3–12) → Hard, dense, form colored compounds.
🔹 Explanation: Metals like iron, copper, and zinc are strong and heavy. Their compounds often have bright colors (like blue copper sulfate). - Boron family (Group 13) → Includes metals and metalloids.
🔹 Explanation: This group starts with boron (a metalloid) and includes metals like aluminum and gallium. - Carbon family (Group 14) → Carbon, silicon, tin, etc.
🔹 Explanation: This group has a mix of non-metals (carbon), metalloids (silicon), and metals (tin, lead). - Nitrogen family (Group 15) → Nitrogen, phosphorus, etc.
🔹 Explanation: These elements are important for life. Nitrogen makes proteins and phosphorus is in bones and DNA. - Oxygen family (Group 16) → Oxygen, sulfur, etc.
🔹 Explanation: Oxygen is essential for breathing, sulfur is used in medicines and rubber. - Halogens (Group 17) → Very reactive non-metals, form salts.
🔹 Explanation: Fluorine, chlorine, etc. react easily with metals to make salts (e.g., NaCl = common salt). - Noble gases (Group 18) → Chemically inert gases, very stable.
🔹 Explanation: Helium, neon, and argon do not normally react with other elements because their atoms are already stable.
Group-wise Names
- Group 1 → Alkali metals (Li, Na, K, Rb, Cs, Fr)
- Group 2 → Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra)
- Groups 3–12 → Transition metals (Sc, Ti, V, Cr … to Zn, plus the rest)
- Group 13 → Boron family (B, Al, Ga, In, Tl)
- Group 14 → Carbon family (C, Si, Ge, Sn, Pb)
- Group 15 → Nitrogen family / Pnictogens (N, P, As, Sb, Bi)
- Group 16 → Oxygen family / Chalcogens (O, S, Se, Te, Po)
- Group 17 → Halogens (F, Cl, Br, I, At, Ts)
- Group 18 → Noble gases (He, Ne, Ar, Kr, Xe, Rn, Og)
3. By Periods (Horizontal Rows)
- There are 7 periods.
🔹 Explanation: The periodic table has 7 rows, each called a period. - As you move from left to right → metallic character decreases, non-metallic character increases.
🔹 Explanation: On the left side you find reactive metals, and as you move right, elements become less metallic and more non-metallic (like oxygen, fluorine).
4. By Blocks (Based on Electron Arrangement)
- s-block → Groups 1 and 2.
🔹 Explanation: The outermost electrons of these elements are in the s-orbital. Example: Sodium, calcium. - p-block → Groups 13 to 18.
🔹 Explanation: Their outermost electrons are in the p-orbital. Example: Oxygen, chlorine. - d-block → Transition metals.
🔹 Explanation: These elements have outermost electrons filling the d-orbital. Example: Iron, copper. - f-block → Inner transition elements (lanthanides and actinides).
🔹 Explanation: These elements have electrons in the f-orbital. They are placed separately at the bottom. Example: Uranium, cerium.
5. Special Groups
- Representative elements → s-block and p-block (except noble gases).
🔹 Explanation: These are the main group elements that show a wide range of properties. - Transition elements → d-block.
🔹 Explanation: These are the metals in the middle section (hard, form colored ions). - Inner transition elements → f-block, include rare earths and radioactive elements.
🔹 Explanation: Elements like thorium and uranium are radioactive, while lanthanides are rare-earth metals used in magnets and electronics
Group 1 → Alkali Metals
alkali metals are a group of highly reactive chemical elements in Group 1 of the periodic table, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They are silvery-white, soft metals that readily lose their single outermost electron to form +1 ions. Their name comes from their strong reaction with water to form soluble, basic compounds called alkalis (e.g., sodium hydroxide, or lye).
Key Characteristics
- Location: Group 1 (first column) of the periodic table.
- Reactivity: Extremely high reactivity, especially with water, due to their single valence electron.
- Electronic Configuration: All have one electron in their outermost shell (ns¹ configuration).
- Formation of Alkalis: When reacted with water, they form strong bases, or alkalis, which neutralize acids.
- Physical Properties: They are shiny, soft, and possess excellent electrical and thermal conductivity.
- Low Melting Points: Compared to other metals, they have relatively low melting points.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Alkali metals (Li, Na, K, etc.)
- Trick: “Ali” = Alkali.
- Daily Example: Sodium (Na) is in common salt (NaCl). Potassium (K) is in bananas for health.
- Why called Alkali? They form strong bases (alkalis) when reacting with water.
Group 2 → Alkaline Earth Metals
alkaline earth metals are the Group 2 elements on the periodic table—beryllium, magnesium, calcium, strontium, barium, and radium. They are defined by their chemical properties, including their tendency to lose two valence electrons to form positive ions, their shiny, silvery appearance, and their reactivity, which is generally lower than alkali metals but higher than main group elements in later groups.
Key Characteristics
- Location on the Periodic Table: They are the second column (Group 2) of the periodic table, following the alkali metals.
- Valence Electrons: Each alkaline earth metal has two electrons in its outermost shell (s-orbital), which they easily lose to form a stable, doubly charged positive ion (cation).
- Physical Properties: They are typically lustrous, shiny, silvery-white metals that are soft to semi-soft, though beryllium and magnesium are harder and less reactive than the heavier alkaline earth metals.
- Reactivity: They are reactive metals, but less so than the alkali metals. They react with oxygen and water to form compounds.
- Historical Naming: The “earth” in their name comes from the Middle Ages, when “earths” referred to substances that were insoluble in water and didn’t change when heated.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Alkaline earth metals (Be, Mg, Ca, etc.)
- Trick: “Always” = Alkaline.
- Daily Example: Calcium (Ca) is in milk and bones. Magnesium (Mg) is in medicines and green plants (chlorophyll).
- Why important? They are “earthy” minerals found in soil and rocks.
Groups 3–12 → Transition Metals
transition metals are d-block elements (found in groups 3-12 of the periodic table) characterized by an incompletely filled d-subshell, either in their ground state or in one of their common oxidation states. These elements exhibit properties such as forming variable oxidation states, creating colored compounds, acting as catalysts, and forming alloys.
Key Characteristics
Formation of Alloys: They readily form alloys with other metals, creating strong and useful materials.
Incompletely Filled d-orbitals: The defining feature of transition metals is their partially filled d-subshell. According to the International Union of Pure and Applied Chemistry (IUPAC) definition, this applies to elements with a d-subshell that is partially filled, or those that can form stable cations with an incompletely filled d-orbital.
d-block Elements: They occupy the central block of the periodic table, situated between the s-block (Groups 1 and 2) and the p-block (Groups 13 to 18).
Variable Oxidation States: Transition metals can lose varying numbers of electrons to form ions with different charges, a property that contributes to their diverse chemistry.
Formation of Colored Compounds: The presence of unpaired electrons in their incompletely filled d-orbitals allows transition metals to absorb certain wavelengths of light, resulting in colored compounds.
Catalytic Activity: Many transition metals are excellent catalysts, facilitating chemical reactions by adopting multiple oxidation states and forming coordination complexes.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Transition metals (Fe, Cu, Zn, etc.)
- Trick: “Train in the Middle.” (They lie in the middle of the periodic table).
- Daily Example: Iron (Fe) is used in bridges, buildings. Copper (Cu) is used in electrical wires. Zinc (Zn) is in batteries and medicine.
- Special property: Their compounds are often colored (like blue copper sulfate).
Group 13 → Boron Family
the Boron family, also known as Group 13 of the periodic table, refers to the elements boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh). These elements share common characteristics, including having three valence electrons and demonstrating a trend from metalloid (boron) to metallic character as you move down the group.
Key Characteristics
- Location: They are found in the p-block of the periodic table.
- Electronic Configuration: Each element in the group has the general electronic configuration of ns² np¹.
- Valence Electrons: All members of the family have three electrons in their outermost shell.
- Metalloid to Metallic Character: Boron is the only non-metal (a metalloid), while all other elements in the family are metals.
- Oxidation States: They typically exhibit an oxidation state of +3, though some, like indium, gallium, and thallium, can also show a +1 oxidation state.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Boron family (B, Al, Ga, etc.)
- Trick: “Boys” = Boron family.
- Daily Example: Aluminum (Al) is in foil wraps and utensils. Boron (B) is in borax used for cleaning.
- Fun fact: Aluminum cans are used for cold drinks.
Group 14 → Carbon Family
the Carbon Family, also known as Group 14 or tetrels, refers to the group of elements on the periodic table that includes Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb), and Flerovium (Fl). These elements share similar valence electron configurations of ns²np² and exhibit trends in properties, such as increasing metallic character and differing oxidation states, down the group.
Key Characteristics
- Valence Electrons: All elements in Group 14 have four valence electrons, giving them a general electronic configuration of ns²np².
- Metallic Character: The family shows a transition from non-metallic (Carbon) to metalloid (Silicon and Germanium) to metallic (Tin and Lead).
- Oxidation States: Carbon and Silicon typically show a +4 oxidation state. Heavier elements like Germanium, Tin, and Lead show an increasing tendency to form +2 oxidation states due to the inert pair effect, where the ns² electrons become less involved in bonding.
- Unique Properties: Carbon, the first element in the group, exhibits anomalous properties and is the backbone of all known life, forming a vast array of compounds.
- Other Names: This family is also referred to as tetrels or tetragens due to the four valence electrons, or crystallogens, which highlights the importance of elements like silicon in crystal formation, particularly in the electronics industry.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Carbon family (C, Si, Sn, Pb, etc.)
- Trick: “Carrying” = Carbon family.
- Daily Example: Carbon (C) is in coal, pencils (graphite), and diamonds. Silicon (Si) is used in computer chips. Tin (Sn) is used to coat cans. Lead (Pb) was in old pencils and batteries.
Group 15 → Nitrogen Family (Pnictogens)
The “Pnictogen” group because the name comes from the Greek word “pnigein,” which means “to choke” or “to stifle”. This refers to the hazardous, suffocating nature of molecular nitrogen (N₂), a prominent member of this group, especially in enclosed, oxygen-deficient spaces. Because of this property, Group 15 elements are also known as the “nitrogen family”.
Key characteristics of Pnictogens:
- Group 15 elements: This group contains Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi).
- “To choke” or “to stifle”: The name “Pnictogen” highlights the toxic and suffocating nature of some elements in this family, such as arsenic, which can be poisonous.
- Five valence electrons: Each element in this group has five electrons in their outermost shell, making them relatively electron-deficient and prone to forming compounds.
- Trend in metallicity: While nitrogen and phosphorus are non-metals, arsenic and antimony are metalloids, and bismuth is a metal, showing an increasing metallic character down the group.
- Biological and Material Significance: Nitrogen and phosphorus are essential for life, while elements like bismuth have found uses in various alloys and compounds.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Nitrogen family (N, P, As, Sb, Bi)
- Trick: “New” = Nitrogen family.
- Daily Example: Nitrogen (N) is 78% of air, used in fertilizers. Phosphorus (P) is in matchsticks and bones.
- Why special? Without nitrogen, plants cannot grow!
Group 16 → Oxygen Family (Chalcogens)
In chemistry, the Oxygen Family, or Chalcogens, are the elements in Group 16 of the periodic table: oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). The term “chalcogen” comes from the Greek words for “ore-forming” because these elements, especially in the form of oxides and sulfides, are commonly found in metal ores and are a significant source of many metals.
Key Characteristics:
- Group 16 Elements: They share a general outer electronic configuration of ns²np⁴, indicating they have six valence electrons.
- Ore Formation: They readily form compounds with metals, such as oxides (like iron oxide Fe₂O₃) and sulfides (like copper sulfide CuS), which are crucial sources of metals.
- Reactivity: They are highly reactive, with a strong tendency to gain two electrons to achieve a stable noble gas configuration, leading to a common -2 oxidation state.
- Abundance: Oxygen is the most abundant element in the Earth’s crust and essential for life.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Oxygen family (O, S, Se, etc.)
- Trick: “Old” = Oxygen family.
- Daily Example: Oxygen (O) is for breathing. Sulfur (S) is used in fireworks and medicines. Selenium (Se) is in solar panels.
- Why called Chalcogens? They are “ore formers” (found in ores).
Group 17 → Halogens
halogens are the highly reactive, nonmetal elements in Group 17 of the periodic table: fluorine, chlorine, bromine, iodine, and astatine. The term “halogen” comes from Greek roots meaning “salt-forming” because these elements readily react with metals to produce salts. They are characterized by having seven valence electrons, which gives them a strong tendency to gain one more electron to achieve a noble gas electron configuration, making them highly electronegative and reactive.
Key Characteristics
- Reactivity: Halogens are among the most reactive nonmetals due to their high electronegativity.
- Electron Configuration: They all have seven electrons in their outermost shell (ns²np⁵ configuration), which is just one electron short of a stable noble gas configuration.
- Salt Formation: They react with metals to form various types of salts, such as sodium chloride (table salt).
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Halogens (F, Cl, Br, I, At)
- Trick: “Heavy” = Halogens.
- Daily Example: Fluorine (F) is in toothpaste. Chlorine (Cl) is in bleaching powder and swimming pool water. Iodine (I) is in salt to keep our thyroid gland healthy.
- Fun fact: Halogen means “salt producer.”
Group 18 → Noble Gases
noble gases are elements in Group 18 of the periodic table, known for their extremely low chemical reactivity and stable, fully filled outer electron shells, which contain a complete set of electrons (two for helium and eight for others). These characteristics make them colorless, odorless, tasteless, nonflammable, and largely unreactive under normal conditions, existing as monatomic gases. The elements in this group include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and the synthetic oganesson (Og).
Key Characteristics
- Location: Noble gases are found in Group 18 (or formerly Group 0) of the periodic table.
- Reactivity: They exhibit very low chemical reactivity because their valence (outer) electron shells are complete, often described as a stable electron configuration or “octet rule”.
- Physical Properties: They are colorless, odorless, tasteless, and exist as monatomic gases under standard conditions.
- Stability: Their full electron shells make them highly stable and unreactive with other elements.
Memory tricks (mnemonics) for remembering the group names in order.
- Name: Noble gases (He, Ne, Ar, Kr, Xe, Rn, Og)
- Trick: “Notes” = Noble gases.
- Daily Example: Helium (He) is used in balloons. Neon (Ne) is in neon signboards. Argon (Ar) is used in light bulbs.
- Why called Noble? They are “noble” because they don’t react with others (inert).
Questions On Classification of elements in the periodic table
🟡 Very Short Answer (1 Mark Questions)
- What are Group 1 elements called?
- Name one daily life use of sodium.
- Which group is known as “noble gases”?
- Write an example of a metalloid.
- Which group elements are found in bones and teeth?
🔵 Short Answer (2–3 Marks Questions)
- Why are Group 1 elements called alkali metals? Give one example.
- Write two uses of noble gases in daily life.
- Mention two differences between metals and non-metals.
- Why are halogens called “salt formers”? Give an example.
- Name the element used in:
(a) matchsticks
(b) computer chips
(c) toothpaste
🔴 Long Answer (4–5 Marks Questions)
- Write the names of Groups 13 to 18 along with one element each.
- Explain why transition metals are called “colored element makers” with examples.
- Describe the importance of calcium, phosphorus, and nitrogen in our daily life.
- Write the memory trick for remembering all groups and explain it.
- With examples, explain how Group 1, Group 2, and Group 17 elements are connected to our everyday life.
Answers On Classification of elements in the periodic table
🟡 Very Short Answer (1 Mark Questions)
- Group 1 elements are called Alkali metals.
- Sodium is used in making common salt (NaCl).
- Group 18 elements are called Noble gases.
- Silicon (Si) is an example of a metalloid.
- Group 2 (Alkaline earth metals, e.g., calcium) are found in bones and teeth.
🔵 Short Answer (2–3 Marks Questions)
- Group 1 elements are called alkali metals because they form strong alkaline solutions when they react with water. Example: Sodium reacts with water to form sodium hydroxide (NaOH).
- Uses of noble gases:
- Helium is used in balloons.
- Neon is used in advertising signboards.
- Differences between metals and non-metals:
- Metals are shiny, while non-metals are dull.
- Metals conduct heat and electricity, while non-metals are poor conductors.
- Halogens are called “salt formers” because they react with metals to form salts. Example: Sodium + Chlorine → Sodium chloride (NaCl).
- Uses of elements:
- (a) Phosphorus is used in matchsticks.
- (b) Silicon is used in computer chips.
- (c) Fluorine (as fluoride) is used in toothpaste.
🔴 Long Answer (4–5 Marks Questions)
- Groups 13 to 18 names with examples:
- Group 13 → Boron family (example: Aluminum).
- Group 14 → Carbon family (example: Carbon).
- Group 15 → Nitrogen family / Pnictogens (example: Nitrogen).
- Group 16 → Oxygen family / Chalcogens (example: Oxygen).
- Group 17 → Halogens (example: Chlorine).
- Group 18 → Noble gases (example: Helium).
- Transition metals are called “colored element makers” because their compounds often show bright colors.
- Example: Copper sulfate is blue.
- Potassium dichromate is orange.
- Nickel compounds are green.
- Importance in daily life:
- Calcium (Group 2) → Strengthens bones and teeth, found in milk.
- Phosphorus (Group 15) → Used in DNA and matchsticks.
- Nitrogen (Group 15) → Essential for plant growth, present in fertilizers.
- Memory trick:
👉 “Ali Always Trains Boys Carrying New Old Heavy Notes.”
- Ali = Alkali metals (Group 1)
- Always = Alkaline earth metals (Group 2)
- Trains = Transition metals (Groups 3–12)
- Boys = Boron family (Group 13)
- Carrying = Carbon family (Group 14)
- New = Nitrogen family (Group 15)
- Old = Oxygen family (Group 16)
- Heavy = Halogens (Group 17)
- Notes = Noble gases (Group 18)
- Everyday life uses:
- Group 1 (Alkali metals): Sodium in table salt, potassium in bananas.
- Group 2 (Alkaline earth metals): Calcium in bones, magnesium in medicines.
- Group 17 (Halogens): Chlorine in water purification, fluorine in toothpaste, iodine in salt.